Methodology of process validation
Process validation may be sub-categories into three phases:
1. process design
2. process qualification
3. Continuous process verification
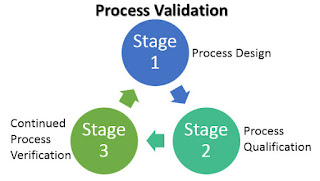 |
Fig: Cycle of process validation |
1. Process design:
The objective of this phase is to design a process appropriate for routine commercial manufacturing that can consistently produce a produce that meets its quality attributes.
2. Process qualification:
During this phase, the process design is evaluated to determine the process is capable of reproducing the commercial manufacturing. This phase to be done in two stages:
A. Part-1: Design of the facility and qualification of the equipment and utilities.
B. Part-2: Process performance qualification (PPQ): cGMP complaints procedure must be followed during this stages. The need of training to be assessed prior to starting up of PPQ batches. Process for new product & existing products to be qualified based on current version of the process validation master plan (PVMP).
3. Continuous process verification:
The process parameters & quality attributes are monitoring under this phase continuously, at the level that had been established during process qualification phase.
Continuous process verification to be performed in steps as given below:-
ü Identification of CPP & CQA for Continuous process verification.
ü Continuous process verification throughout the life cycle of the product.
ü Annual product quality review.


Comments
Post a Comment